1. Grant R. Single layer closure with V-Loc™ 180 device compared to double layer closure using standard suture methods. Internal V-Loc™
180 Absorbable Wound Closure Device Time Study. New York-Presbyterian Hospital, Argent global Services.
2. UTSW, Brown S. Utilization of a porcine model to demonstrate the efficacy of an absorbable barbed suture for dermal closure. 2010;
Internally sponsored study.
3. Time motion study – comparison of wound closure time using conventional techniques and knotless, self-anchoring surgical sutures in
ex-vivo porcine model for both single layer and double layer closure in all closure techniques. Royal College of Surgeons, London, UK.
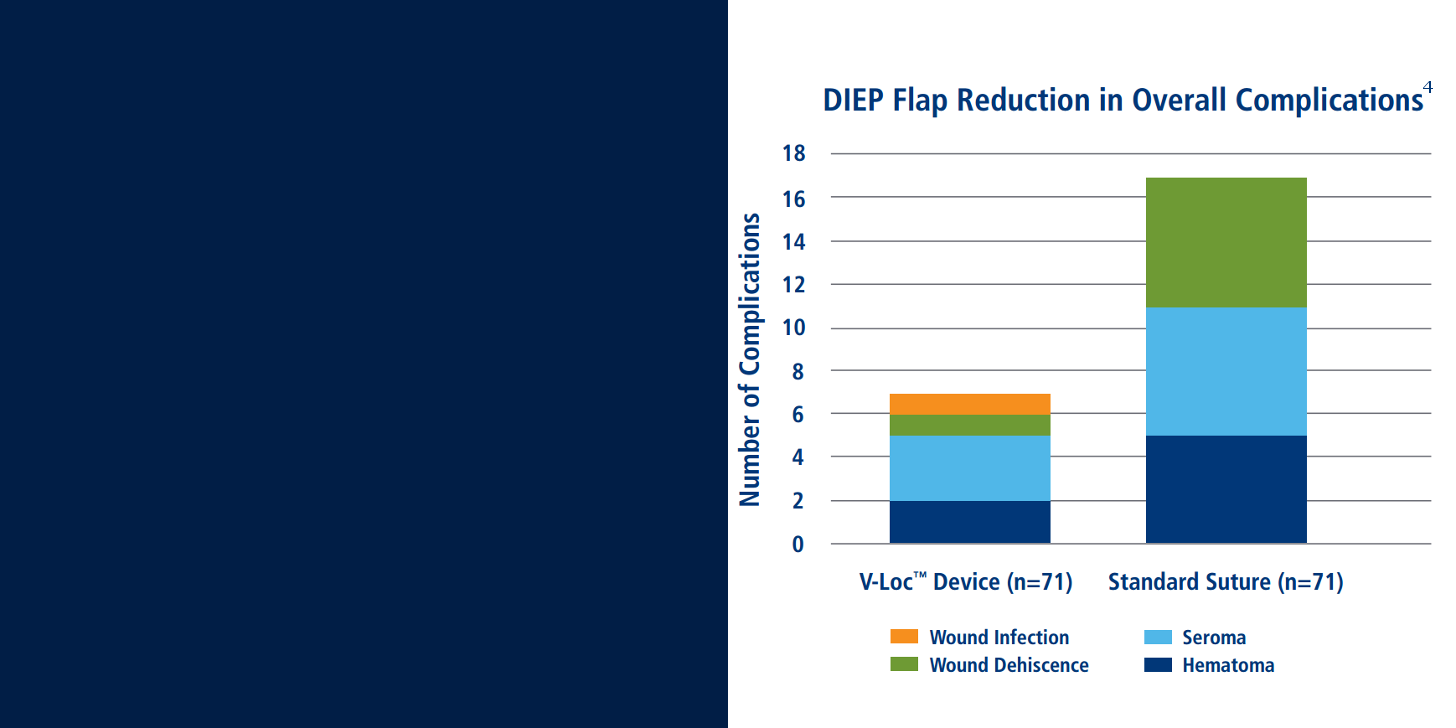
4. De Blacam et al. “Early Experience With Barbed Sutures for Abdominal Closure in Deep Inferior Epigastric
Perforator Flap Breast Reconstruction” Presented at the New England Society of Plastic and Reconstructive
Surgeons Meeting, Brewster, MA June 2011. Published: Eplasty.com, 5.2012.
5. Capla, JM; A prospective, randomized study to evaluate dermal closure with an absorbable barbed suture (V-Loc™ 90 device or V-Loc™ 180 device) as compared to a conventional.
6. Alessandri F, Remorgida V, Venturini PL, Ferrero S, et al. Unidirectional barbed suture versus continuous
suture with intracorporeal knots in laparoscopic myomectomy: a randomized study. Journal of minimally
invasive gynecology, 17(6), 725-9, 11-12.2011.
7. Based on internal benchtop test report #SHP-024. P value <0.001. February 2016.
8. Based on internal benchtop test report #SHP-024. P value <0.001. February 2016.
9. Based on internal benchtop test report #SHP-017-1. P value <0.001. March 2015.
 This site uses cookies to store information on your computer. Some are essential to make our site work; others help us improve the user experience. By using the site, you consent to the placement of these cookies. Read our privacy statement to learn more.
This site uses cookies to store information on your computer. Some are essential to make our site work; others help us improve the user experience. By using the site, you consent to the placement of these cookies. Read our privacy statement to learn more.